TB-Feron ELISA 48 Wells – SD BIOSENSOR
Intended Use
STANDARD E TB-Feron ELISA is developed based on the IGRA (Interferon Gamma Releasing Assay) method for the diagnosis of latent tuberculosis infection
Advantage
- Sensitive and specific IGRA test
- Unlike the classic way of tuberculin test (TST), TB-Feron ELISA only needs one clinic visit
- Not affected by Bacille-Calmette Guerin (BCG) vaccinations
₹33,500.00 ₹50,000.00
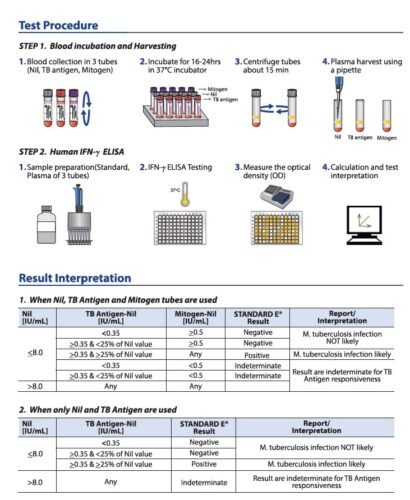
Introduction to Standard E TB-Feron ELISA
The Standard E TB-Feron ELISA is an advanced diagnostic tool developed based on the Interferon Gamma Releasing Assay (IGRA) method. This innovative product is designed to detect latent tuberculosis infection (LTBI) with high accuracy and reliability.
How IGRA Method Enhances Diagnosis
The IGRA method employed in the Standard E TB-Feron ELISA measures the release of interferon-gamma (IFN-γ) when blood samples are exposed to specific tuberculosis antigens. This method is particularly effective in distinguishing between latent and active tuberculosis, making it a vital tool in the global effort to control TB.
Advantages of Using Standard E TB-Feron ELISA
One of the primary benefits of the Standard E TB-Feron ELISA is its high specificity and sensitivity. Unlike traditional tuberculin skin tests, this ELISA-based assay minimizes the risk of false-positive results, especially in individuals vaccinated with Bacillus Calmette-Guérin (BCG). Additionally, the test provides rapid results, facilitating timely decision-making and treatment planning.
Clinical Applications and Utility
The Standard E TB-Feron ELISA is suitable for various clinical settings, including hospitals, clinics, and research laboratories. Its ability to accurately diagnose latent tuberculosis infection makes it an essential tool for healthcare providers aiming to prevent the progression of TB in at-risk populations.
Conclusion
In summary, the Standard E TB-Feron ELISA represents a significant advancement in the diagnosis of latent tuberculosis infection. By leveraging the IGRA method, it offers healthcare professionals a reliable and efficient means to identify and manage LTBI, contributing to the broader goal of tuberculosis eradication.

| Weight | 1.00 kg |
|---|---|
| Dimensions | 23 × 15 × 10 cm |
| GTIN | |
| BRANDS |

You must be logged in to post a review.








Reviews
There are no reviews yet